Description
Quick Details
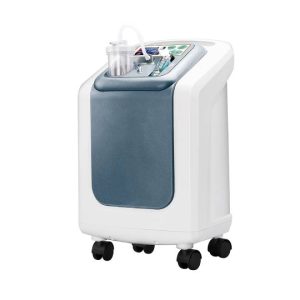
1. Fashionable appearance and small size;
2. lightweight: easy move way
3. Original design and building
4. Self. patented core components
5. It can produce mist gas with much smaller diameter, than supersonic nebulizer.
6. Low Noise :≤58dB (A)
7. Energy-saving and economical: working more than 11 hour's just need 1 degree eletricity.
Packaging & Delivery
| Packaging detail:Standard export package Delivery detail:within 7-10 workdays after receipt of payment |
Specifications
Medical Air-compressor Nebulizer
Before using the nebulizer, please read user manual carefully
User Manual
Introduction
Thank you for purchasing our Compressor Nebulizer.
The Device includes a nebulzier cup,an air tube,a mouthpiece,an user manual and a compressor with air pump that generates compressed air.The nebulizer kit converts the liquid medication into a fine aerosol which is inhaled by the patient via a mouthpiece for the treatment of respiratory disorders.
The Compressor Nebulizer is a medical device.Operate the device only as instructed by your doctor and/or respiratory therapist.
The device fulfills the provisions of the EC directive 93/42/EEC(Medical Device Directive)and the European Standard EN 13544-1:2007,Respiratory therapy equipment-part1:Nebulizing systems and their components.
The user can use accessories made by other manufacturer,such as masks and the mouthpieces,which matched the nebulzier kit and must comply with ISO10993-1.
Intended Use
Intended Use The device is intended to be used for inhaling medication for respiratory disorders.
Intended User Legally certified medical experts,such as doctor,nurse and the therapist or health care personnel or patient under the guidance of qualified medical experts for the home treatment.
Intended Patients The device should not used by patient who are unconscious or are not breathing spontaneously.
Precautions for use Warnings and cautions described in the Instruction manual should be observed.
Symbol Use
Meanings of Symbols
Signs Notes on the signs
Type BF Applied Part
Class II equipment
Refer to instruction manual/ booklet
Manufacturer
Date of manufacturer
Authorized Representative in the European community
Batch code
serial number
Indicates a potentially hazardous situation which, if not avoided, could result in death or serious injury??
Indicates a potentially hazardous situation which, if notavoided, may result in minor or moderate injury to the user or patient or damage to the equipment or other property.
The marking of electrical and electronics devices according to Directive 2002/96/EC. The device, accessories and the packaging have to be disposed of waste correctly at the end of the usage.Please follow Local Ordinances or Regulations for disposal
CE marking
Protected against solid foreign objects of 12.5mm diameter and greater.
Temperature limit
Humidity limitation
Atmospheric pressure limitation

Important Safety Notes
Read all the information in the user manual and any other literature included in the box before using the device.
To assure the correct use of the device,basic safety notes should always be followed including the precautions listed below:
Warning
Indicate a potentiality hazardous situation which,if not avoided,could result in serious injury.
(Usage)
- For type,dose,and regime of medication,follow the instructions of your doctor or respiratory therapist.
- Do not use only water in the nebulzier for nebulizing purposes.
- If you feel anything unusual during use,stop using the device immediately and consult your doctor.
- Do not use the device where it may be exposed to flammable gas.
- Make sure that the nebulizer kit is clean before use.
- Do not operate the device in the presence of any anaesthetic,inflammable mixtures or oxygen.
- Keep the device out of the reach of unsupervised infants and children.The device may contain small pieces that can be swallowed.
- Store the device and the accessories in a clean location.
- Do not store the air tube while there is moisture or medication remaining inside it.Do not use or store the device where it may be exposed to noxious fumes or volatile substances.
- Do not cover the compressor with a blanket or tower etc.during use.
- Always dispose of any remaining medication after use,and use fresh medication each time.
- Do not use in anaesthetic or ventilator breathing circuits.
- Mouthpiece,mask must comply with ISO10993-1.
- Any components,if damaged,is not user serviceable,need to be repaired by qualified service personnel.
Important Safety Notes-1
(Risk of electrical shock)
The compressor are not waterproof. Do not spill water,or other liquids,on these parts.if liquid does spill on these parts, immediately unplug the plug.
Do not immerse the compressor(main unit) in water or other liquid.
Do not use or store the device in humid locations, such as bathroom.
Indicate a potentially hazardous situation which,if not avoided,may result in minor or moderate Injury,or physical damage.
Provide close supervision when the device is used by,on,or near children or invalids.
Make sure that the parts are attached correctly.
Make sure that the air filter is correctly attached.
Do not spill liquid or medication on the compressor.
Do not use or store the device while the air tubes is creased.
Do not add more that 10ml of medication to the medication tank.
Important Safety Notes-2
- Do not leave the device unattended infants or persons who cannot express their consent.
- Do not subject the device or the parts to any strong shocks such as dropping the device on the floor.
- Do not disassemble or attempt to repair the compressor.
- Do not block the air filter cover.
- Do not leave the device while sleeping or if drowsy.
- The compressor may become hot during operation.
- Do not touch the compressor or other than necessary operation such as turning off the power while nebulizing.
- To avoid the medication residue on the face,be sure to wipe the face after removing the mask.
- Do not use a damaged nebulizer kit,mouthpiece or nebulziation mask.
- Do not block the slit between the cap and the inhalation air inlet.
- Do not use a microwave oven or hair dryer to dry the device to the parts
- Approved for human use only.
(Risk of electrical shock)
Always remove the plug after use and before cleaning the unit.
Changes or modifications will void the user warranty.
General Safety precautions:
Inspect the device and the parts before using them each time,chant check that there are no problems.
When using the device,there will be some noise and vibration caused by the pump in the compressor.there will also be some noise cause by the emission of compressed air from the nebulzier kit,which is normal and does not indicates a malfunction.
Operate the device only as intended.Do not use the device for any other purpose.
Make sure that the air tube is securely attached to the compressor(main unit).and the nebulizer kit and does not come loose.
Know your device
THE UNIT STANDARD FITTINGS INCLUDE:
(1)Aerosol therapy unit
(2)Air inlet
(3)Air filter
(4)On/off switch
(5)Power cord
(6)Connection tube
(7)Nebulizer Cup
(8)Audit mask
(9)Child mask

Accessories of the unit
1.Connection tube 1PC
2.Nebulizer Cup 1PC
3Audit mask 1PC
4.Child mask 1PC
TREATMENT OF THE WHOLE RESPIRATORY TRACT
1.INSTRUCTION FOR NEBULIZER
1 Plug the power cord into a power socket corresponding to the voltage of the unit.
2 Carefully wash your hands before going ahead with inhalation therapy.
3 Open the nebulizer by turning the upper part
4 Pour the drug prescribed by the physician into the lower part .Close the nebulizer by turning the upper part
5 Connect the accessories as shown in the “Assembly diagram”. The upper cap provided with your nebulizer makes it possible to deliver the medication.
6 Sit comfortably ,holding the nebulizer in your hands ,place the mouthpiece over your mouth or
use the mask ,Start the unit by means of switch an breathe in and out deeply .
7 .When the treatment is finished, turn off and unplug the unit.
8. It may happen that after a treatment session visible humidity deposits form in the tube: disconnect the tube from the nebulizer and let it dry using the compressor air flow, as this prevents mould from growing in the tube.
2.CLEANING
Before undertaking any cleaning operation, switch off and unplug the compressor.
Use a damp cloth to wipe down the outside of the compressor at least once each month (or more if necessary). Do not use abrasive cleaners.
Check the filter at least once each week. Replace the filter once a month or when dirty.
In order to prevent the cross-infection, the nebulzier parts (connection tube, nebulizer cup, child mask, adult mask) are single-use. For the replaceable nebulizer parts,please contact your dealer.
Only use with the nebulzier parts (connection tube, nebulizer cup, child mask, adult mask) which with the Notified Body CE making.
Unit and exterior of the tube
To clean use only a damp cloth and an antibacterial detergent (non-abrasive and solvent free)
3.AIR FILTERING
The unit is equipped with an air filter which should be replaced when dirty or when its color changes. Do not wash or re-use the used filter. The filter must be regularly replaced to help ensure efficient compressor performance .The filter should be regularly checked. For spare filter contact you dealer or an authorized service center.
To replace the filter
Lift the id and remove the filter using a screwdriver. The filter is designed to remain fixed in its housing.
Use only original accessories
4. Service Life
WARNING!
Risk of Injury or Damage
The product has been tested for the service life stated in this manual. Use of the product beyond this time period may cause injury or product damage.
– ONLY use the product for the service life stated in this manual. DO NOT exceed the service life of the product.
– Perform all maintenance according to the recommended schedule in this manual.

The expected service life of this product is 2000hrs.of operation when used in accordance with the safety instructions, maintenance intervals and correct use, stated in this manual. The effective service life can vary according to frequency and intensity of use. Refer to the Maintenance section.
TECHNICAL SPECIFICATIONS
Model: MSLWHB02
Voltage: 230V/50Hz
Fuse: T 2A-250V
Max pressure: 1.2 bar approx.
Dimensions: 205*168*100L*W*H)MM
Weight: 1.7kg
Sound level (at 1m): 60dB(A) approx.
Continuous use
Nebulizer
Medication minimum capacity 2ml
Medication maximum capacity 8ml
Particle Size:MMAD approximately 5.1 μm (based on EN13544-1)
MMAD=Mass Median Aerodynamic Diameter
Nebulization Rate:≤0.2ml/min
Operating pressure (with nebulizer) 0.60bar approx.
Operating conditions : Temperature :min 5??; max 40??
Air humidity: min 10%; max 95%
Storage conditions : Temperature :min -25??; max 70??
Air humidity: min 10%; max 95%
Transportation conditions:Temperature :min -25??; max 70??
Air humidity: min-25%; max 95%
At atmospheric pressure operating and storage conditions: min700hPa; max 1060hPa
Notes:
Subject to technical modification without prior notice.
The device is produced under our strict quality system.
The device may not work if the temperature and voltage conditions are different to those defined in the specifications.
Performance may vary with drugs such as suspensions or high viscosity.See drug supplier’s data sheet for further details.
**Independently measured according to EN 13544-1.

EMC Statement
This product needs special precautions regarding EMC and needs to be installed and put into service according to the EMC information provided, and this unit can be affected by portable and mobile RF communications equipment.
1) * Do not use a mobile phone or other devices that emit electromagnetic fields, near the unit. This may result in incorrect operation of the unit.
2) Caution: This unit has been thoroughly tested and inspected to assure proper performance and operation!
3) * Caution: this machine should not be used adjacent to or stacked with other equipment and that if adjacent or stacked use is necessary, this machine should be observed to verify normal operation in the configuration in which it will be used.
Guidance and manufacture’s declaration – electromagnetic emission
The DEVICE is intended for use in the electromagnetic environment specified below. The customer of the user of the DEVICE should assure that it is used in such an environment.
Emission test
RF emissions
CISPR 11
Compliance
Group 1
Class B
Electromagnetic environment – guidance
The DEVICE use RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment.
Emission test
RF emission
CISPR 11
Compliance
Class B
Electromagnetic environment – guidance
The DEVICE is suitable for use in all establishments, including domestic establishments and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes.
Guidance and manufacture’s declaration – electromagnetic immunity
The DEVICE is intended for use in the electromagnetic environment specified below. The customer or the user of DEVICE should assure that it is used in such an environment.
Immunity test
Electrostatic discharge (ESD)
IEC 61000-4-2
IEC 60601 test level
±6 kV contact
±8 kV air
Compliance level
±6 kV contact
±8 kV air
Electromagnetic environment – guidance
Floors should be wood, concrete or ceramic tile. If floor are covered with synthetic material, the relative humidity should be at least 30%.
Immunity test
Power frequency (50Hz/60Hz) magnetic field IEC 61000-4-8
IEC 60601 test level
3 A/m
Compliance level
3 A/m
Electromagnetic environment – guidance
Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment.
NOTE UT is the a.c. mains voltage prior to application of the test level.
Guidance and manufacture's declaration–electromagnetic immunity
The DEVICE is intended for use in the electromagnetic environment specified below. The customer or the user of the DEVICE should assure that it is used in such an environment.
Immunity test
Conducted RF IEC 61000-4-6
Radiated RF IEC 61000-4-3
IEC 60601 test level
3 Vrms 150 kHz to 80 MHz
3 V/m 80 MHz to 2.5 GHz
Compliance level
N/A
3 V/m
Electromagnetic environment – guidance
Portable and mobile RF communications equipment should be used no closer to any part of the DEVICE, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter.
Recommended separation distance
Where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in metres (m).
Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey,a should be less than the compliance level in each frequency range.b
Interference may occur in the vicinity of equipment marked with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
a.Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the DEVICE is used exceeds the applicable RF compliance level above, the DEVICE should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the DEVICE.
b.Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Recommended separation distances between portable and mobile RF communications equipment and the DEVICE .
The DEVICE is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the DEVICE can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the DEVICE as recommended below, according to the maximum output power of the communications equipment.
Rated maximum output Separation distance according
power of transmitter to frequency of transmitter
(W) (m)
150 KHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.5 GHz
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in metres (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.














There are no reviews yet.